Services
![HS1[5925].jpg](/sites/uip.pharmacy.uiowa.edu/files/styles/square__1024_x_1024/public/2021-05/HS1%5B5925%5D.jpg?h=8c1344d8&itok=s6EONUW5)
Sterile Manufacturing
![HNS1[5926].jpg](/sites/uip.pharmacy.uiowa.edu/files/styles/square__1024_x_1024/public/2021-05/HNS1%5B5926%5D.jpg?h=b8af5ddf&itok=vvS7Q05S)
Non-Sterile Products
![HA1[5922].jpg](/sites/uip.pharmacy.uiowa.edu/files/styles/square__1024_x_1024/public/2021-05/HA1%5B5922%5D.jpg?h=58c8a5e7&itok=LmLaenNK)
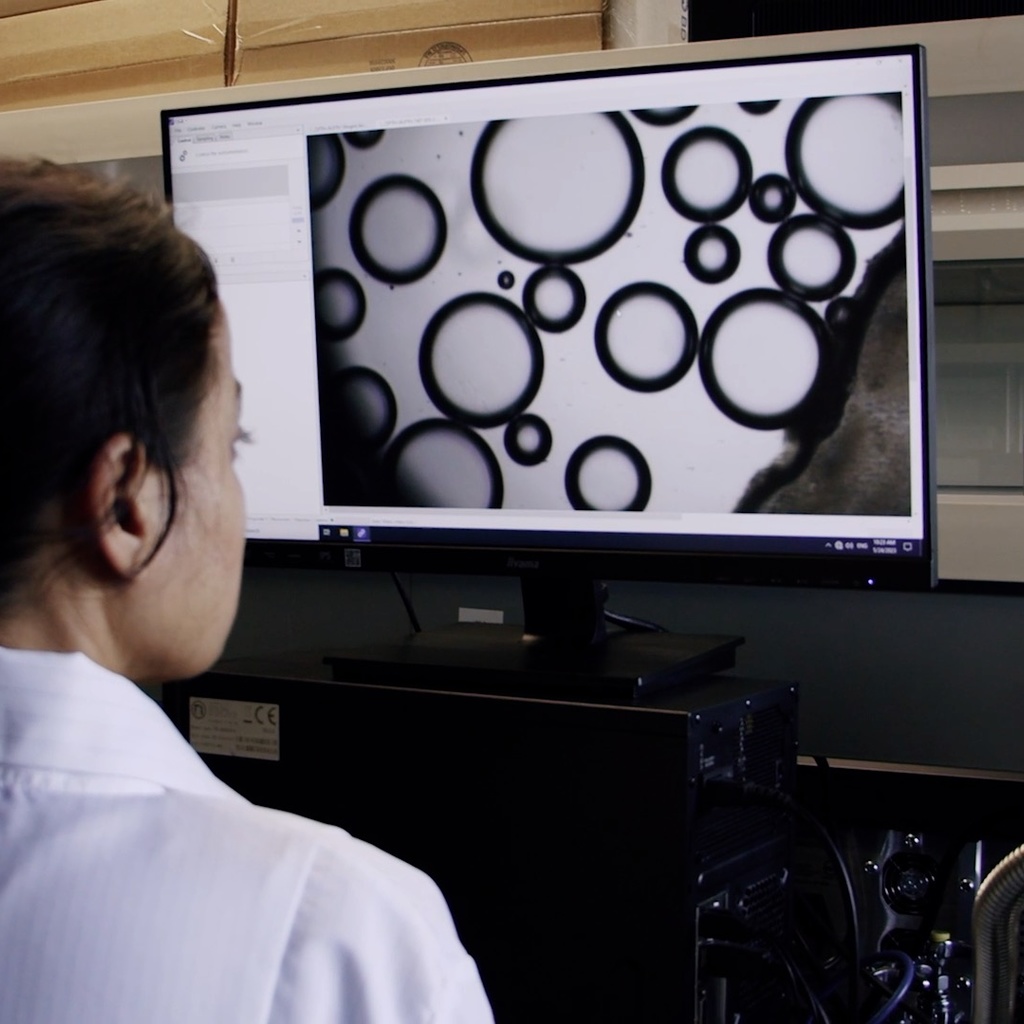
Analytical Services
Visit Our Facilities & Meet Our Experts
News and Announcements

UI Pharmaceuticals Manufactures Advanced Breast Cancer Treatment

From Bench to Bedside: UI Pharmaceuticals Scales Up to Advance Life-Saving Therapies
Exciting Milestone Achieved: Site Registration in South Korea

UI Pharmaceuticals Reaches Milestone

UI Pharmaceuticals Advancing Production
Stay Connected with Industry Leaders
Follow UI Pharmaceuticals on LinkedIn for exclusive insights on pharmaceutical manufacturing, industry innovations, and our latest cGMP clinical trial advancements.
Join our growing network of 2,000+ industry connections!
Pharmaceutical Industry Events
Upcoming Industry Conferences & Exhibition Schedule
Connect with our experts at leading pharmaceutical and biotechnology events! Whether you're exploring drug product manufacturing solutions, have questions about sterile injectables or non-sterile dosage forms, or just want to learn more about our capabilities, stop by our booth to discuss your projects.

2026 Events Coming Soon!
Our business development team is finalizing our attendance at key pharmaceutical and biotechnology conferences in 2026. We're selecting events where we can provide the most value to partners like you.
Be the first to know: Contact us to receive our 2026 event schedule as soon as it's finalized.
2025 Past Events
AAPS 2025 PharmSci 360
📍 Booth 2933, Henry B. Gonzalez Convention Center, San Antonio, TX
📅 Sunday, November 9 — Wednesday, November 12, 2025
2025 Contract Pharma Contracting & Outsourcing Conference
📍 Hyatt Regency, New Brunswick, NJ
📅 Thursday, September 18 — Friday, September 19, 2025
Boulder Peptide Symposium
📍 St. Julien Hotel & Spa, Boulder, CO
📅 Monday, September 15 — Thursday, September 18, 2025
CPHI North America Conference
📍 Booth 3665, Boston Convention & Exhibition Center, Boston, MA
📅 Tuesday, May 20 — Thursday, May 22, 2025
Bio International Convention
📍 Booth 3665, Boston Convention & Exhibition Center, Boston, MA
📅 Monday, June 16 — Thursday, June 19, 2025
Meet Our Experts
Schedule a Personalized Consultation at Industry Events
Don't miss the opportunity to connect with our specialized team at leading pharmaceutical conferences. From innovative drug development solutions to advanced manufacturing partnerships, our experts are ready to help solve your most challenging problems.
Why meet with us:
- Get personalized solutions tailored to your specific challenges
- Discuss strategic partnerships to accelerate your development timeline
- Connect with decision-makers who understand your industry needs
Connect with us in advance to reserve time with our specialists at any upcoming event — Contact Us.